Virus lytic cycle gizmo answers – Welcome to the realm of virology, where we embark on a journey to unravel the intricate details of the virus lytic cycle. Our focus centers on the esteemed Virus Lytic Cycle Gizmo, a valuable tool that empowers us to delve into the fascinating world of viral replication.
Prepare to be captivated as we explore the key stages, mechanisms, and implications of this fundamental biological process.
Virus Lytic Cycle Overview
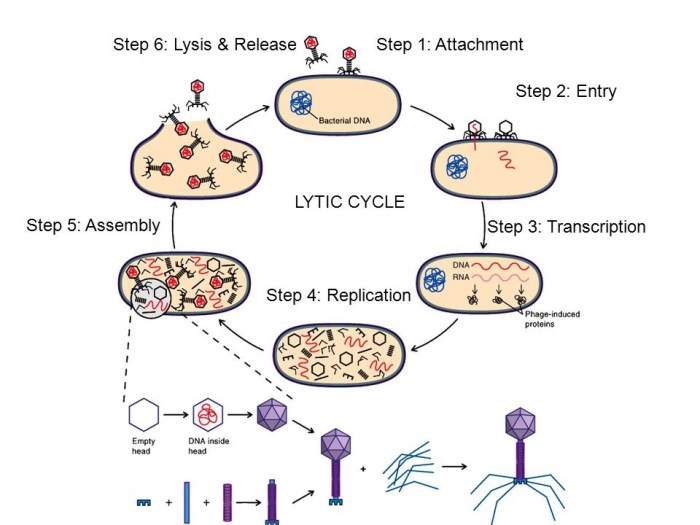
The lytic cycle is a viral replication cycle that results in the destruction of the host cell.
The key stages involved in a lytic cycle are:
- Attachment: The virus attaches to the surface of the host cell.
- Entry: The virus enters the host cell.
- Uncoating: The virus uncoats, releasing its genome into the host cell.
- Replication: The virus genome replicates, producing new copies of the virus.
- Assembly: The new virus particles are assembled.
- Release: The new virus particles are released from the host cell, destroying it.
Examples of viruses that undergo lytic cycles include:
- Bacteriophage T4
- Herpes simplex virus
- Influenza virus
Attachment and Entry
The virus attachment and entry process involves the virus particle binding to specific receptors on the surface of the host cell and then entering the cell.
Virus Attachment
Virus attachment to host cells is a crucial step in the viral life cycle. Viruses have specific proteins on their surface called attachment proteins that bind to complementary receptors on the host cell surface. These receptors can be proteins, carbohydrates, or lipids.
The attachment process is highly specific, as each virus can only bind to a limited range of host cells that express the appropriate receptors. This specificity helps determine the host range of the virus, which is the range of species or cell types that the virus can infect.
Mechanisms of Viral Entry
Once attached to the host cell, viruses use various mechanisms to enter the cell.
- Direct Penetration:Some viruses, such as influenza viruses, have a lipid envelope that fuses with the host cell membrane, allowing the viral nucleocapsid to enter the cell.
- Endocytosis:Many viruses, including HIV and herpesviruses, enter the cell through endocytosis, a process by which the host cell membrane invaginates and engulfs the virus particle.
- Receptor-Mediated Endocytosis:Some viruses, such as adenoviruses, bind to specific receptors on the host cell surface and then enter the cell via receptor-mediated endocytosis, a more specific form of endocytosis.
- Membrane Fusion:Some viruses, such as measles virus, fuse their envelope with the host cell membrane, allowing the viral genome to enter the cell.
Replication and Assembly
The replication of viruses involves several key steps, including the replication of their genetic material and the assembly of new virus particles. These processes rely heavily on the host cell’s machinery, highlighting the parasitic nature of viruses.
Viral Genetic Material Replication
Viruses can replicate their genetic material using various mechanisms depending on their type. DNA viruses utilize host cell enzymes to synthesize new DNA strands, while RNA viruses employ RNA-dependent RNA polymerases to create copies of their RNA genome.
Host Cell Machinery in Virus Replication
Host cell machinery plays a crucial role in virus replication. Viral DNA or RNA polymerases, for instance, require host cell factors for their function. Additionally, viruses often manipulate host cell processes, such as transcription and translation, to favor their own replication.
Assembly of New Virus Particles
Once the viral genetic material has been replicated, new virus particles are assembled within the host cell. The process involves the formation of viral capsids, which encapsulate the genetic material, and the acquisition of viral envelopes, if applicable. Viral assembly often occurs at specific sites within the host cell, such as the cytoplasm or the cell membrane.
Release and Spread: Virus Lytic Cycle Gizmo Answers
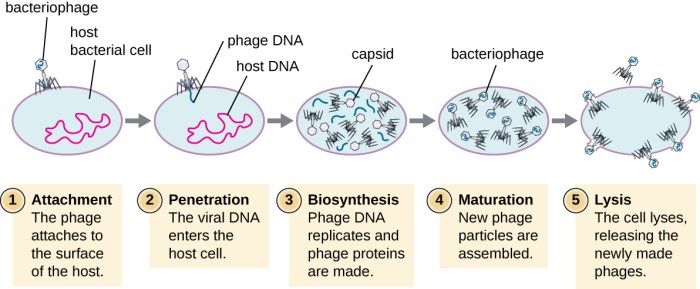
Upon assembly, newly formed virus particles must be released from the host cell to infect new cells. The release mechanisms vary among different viruses.
Budding
- Some viruses, such as HIV and influenza, acquire a lipid envelope from the host cell membrane as they bud out of the cell.
- This budding process does not cause immediate cell lysis.
Cell Lysis
- Other viruses, such as herpes simplex virus and poxviruses, cause the host cell to rupture (lyse) to release the assembled virus particles.
- Cell lysis results in the death of the host cell.
Once released from the host cell, viruses can spread to new cells through various mechanisms:
Contact Transmission
- Viruses can be transmitted through direct contact with an infected individual or contaminated surfaces.
- Examples include respiratory viruses spread through droplets and sexually transmitted viruses.
Airborne Transmission
- Some viruses, such as influenza and measles, can be spread through the air via droplets or aerosols.
- These droplets can remain suspended in the air for extended periods, increasing the risk of infection.
Vector-Borne Transmission, Virus lytic cycle gizmo answers
- Certain viruses, such as dengue and yellow fever, are transmitted by insects or other animals that serve as vectors.
- The vector becomes infected by feeding on an infected host and then transmits the virus to a new host.
The release and spread of viruses have significant implications for host cell health. Cell lysis, caused by some viruses, leads to cell death and tissue damage. Additionally, the inflammatory response triggered by viral infection can further damage host cells and tissues.
Host-Virus Interactions
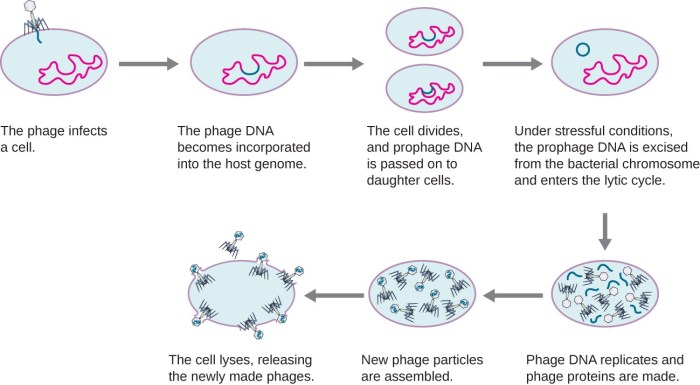
Viral infections pose a significant threat to the host, and the immune system plays a crucial role in defending against these pathogens. The host-virus interaction is a dynamic interplay that involves both the immune system’s response and the virus’s strategies to evade it.
Understanding these interactions is essential for developing effective antiviral therapies and controlling viral infections.
Immune System Response to Viral Infections
Upon infection, the immune system initiates a multifaceted response to eliminate the virus. The innate immune system provides an immediate but non-specific defense, while the adaptive immune system mounts a targeted and long-lasting response.
- Innate Immune Response:Interferons, produced by infected cells, inhibit viral replication. Natural killer cells eliminate virus-infected cells. Phagocytes engulf and destroy viruses.
- Adaptive Immune Response:B cells produce antibodies that neutralize viruses, while cytotoxic T cells kill virus-infected cells.
Viral Evasion of the Immune System
Viruses have evolved diverse strategies to evade the host’s immune defenses:
- Antigenic Variation:Viruses can alter their surface proteins, making them unrecognizable to antibodies.
- Latency:Viruses can enter a dormant state, evading detection by the immune system.
- Immune Suppression:Viruses can produce proteins that suppress the immune response, allowing them to replicate unchecked.
Consequences of Virus-Host Interactions
The outcome of virus-host interactions depends on various factors, including the virulence of the virus, the host’s immune status, and the availability of antiviral treatments.
- Viral Clearance:The immune system successfully eliminates the virus, resulting in recovery from infection.
- Persistent Infection:The virus evades the immune system and establishes a long-term infection, leading to chronic diseases.
- Latency:The virus remains dormant within the host, with the potential for reactivation later.
- Pathogenesis:The virus causes disease symptoms, ranging from mild to severe, depending on the virus and host factors.
Applications and Implications
Understanding the virus lytic cycle has significant implications for public health, disease prevention, and the development of antiviral therapies and vaccines.
Antiviral Therapies
Antiviral therapies aim to disrupt specific stages of the virus lytic cycle, thereby inhibiting viral replication and spread. For example, some antiviral drugs target the viral attachment proteins, preventing them from binding to host cells. Others interfere with viral replication by inhibiting the activity of viral enzymes involved in DNA or RNA synthesis.
Vaccine Development
The virus lytic cycle plays a crucial role in vaccine development. Vaccines stimulate the immune system to produce antibodies that recognize specific viral proteins, such as the attachment protein or the capsid. When a vaccinated individual is exposed to the virus, these antibodies can neutralize the virus and prevent it from infecting host cells.
Public Health and Disease Prevention
Understanding the virus lytic cycle is essential for developing effective public health strategies to prevent and control viral infections. Knowledge of the virus’s transmission mechanisms, host range, and replication rate helps in identifying potential reservoirs and vectors of infection. This information guides public health measures, such as vaccination programs, isolation protocols, and disinfection procedures, aimed at minimizing the spread of viral diseases.
Quick FAQs
What is the significance of the virus lytic cycle?
The virus lytic cycle is a crucial process that enables viruses to replicate and spread, leading to the infection of host cells. Understanding this cycle is essential for developing effective antiviral therapies and vaccines.
How does the Virus Lytic Cycle Gizmo enhance learning?
The Virus Lytic Cycle Gizmo provides an interactive and engaging platform that allows users to visualize and manipulate the different stages of the lytic cycle. This hands-on approach deepens understanding and facilitates the exploration of complex concepts.
What are some real-world applications of understanding the virus lytic cycle?
Knowledge of the virus lytic cycle has practical applications in the development of antiviral drugs, the design of vaccines, and the implementation of public health measures to prevent and control viral infections.